Post-Approval Changes: What Happens After a Drug Gets Approved
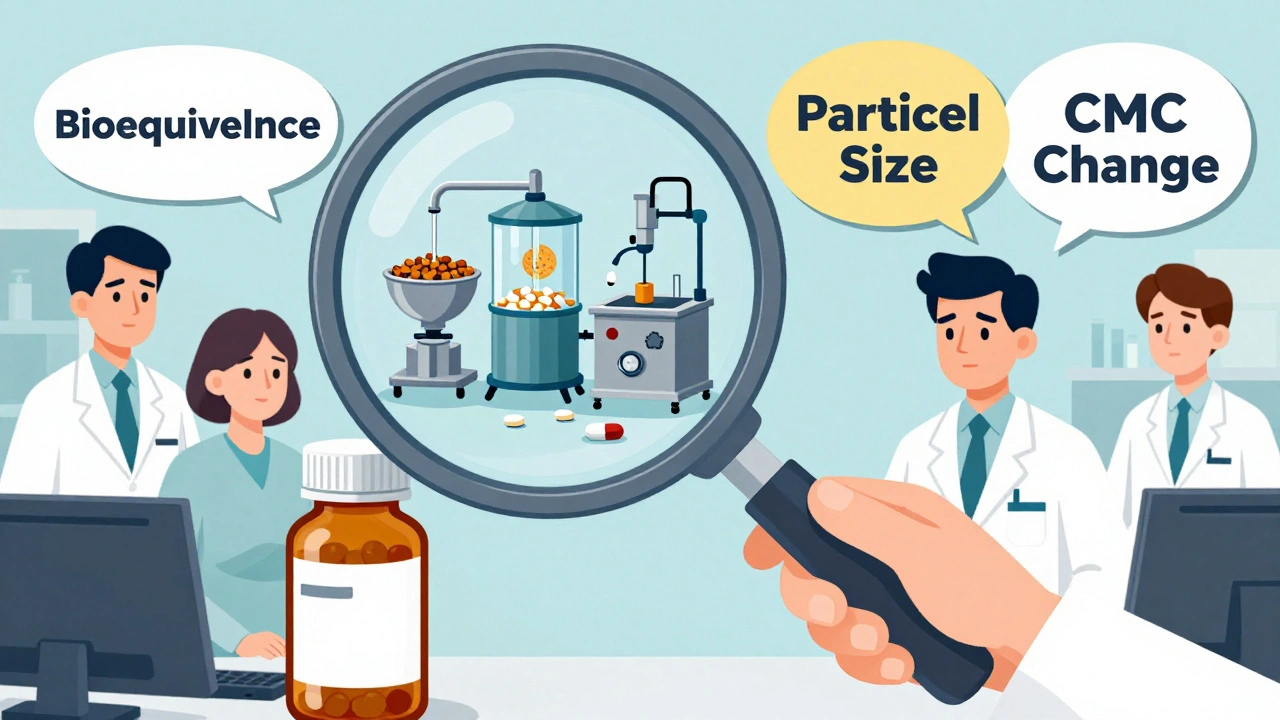
When the FDA approves a drug, it doesn’t mean the story ends. Post-approval changes, modifications made to a drug after it’s on the market, including formulation, manufacturing, or labeling updates. Also known as post-marketing changes, these adjustments can affect how your medication behaves in your body—even if the name and dose stay the same. Many people assume once a drug is approved, it’s frozen in time. But that’s not true. Drugmakers can change things like the inactive ingredients, the shape of a pill, the factory where it’s made, or even how it’s packaged—all without going through a full new approval process. These changes are allowed under FDA rules, as long as they don’t impact safety or effectiveness. But here’s the catch: not all changes are harmless. For drugs with a narrow therapeutic index, medications where tiny differences in dose or absorption can cause serious side effects—like levothyroxine, warfarin, or seizure meds—even small shifts in how the drug is made can throw off your balance.
Generic drug modifications, changes made to generic versions after approval, often to cut costs or improve production are especially common. A pill made in India might switch to a different binder, or a U.S. factory might start using a new machine to press tablets. These aren’t always obvious. You won’t get a notice. The label won’t change. But if you’ve ever felt different after switching to a new batch of generic levothyroxine or your blood thinner, it might not be in your head—it could be in the manufacturing. The FDA tracks these changes through reports and inspections, but they don’t alert patients. That’s why understanding pharmaceutical manufacturing, the process of producing drugs under strict quality controls matters. It’s not just about science—it’s about consistency. A change in the particle size of an active ingredient, or a different drying method during production, can alter how fast your body absorbs the drug. That’s why some people notice side effects after a refill, even if they’re taking the same prescription.
These aren’t just technical details. They’re real health concerns. If you’re on a medication where small changes make a big difference, you need to know what to watch for. Does your energy drop after switching pharmacies? Do you feel dizzy when your pill looks slightly different? These aren’t just coincidences. The post-approval changes behind your meds could be the cause. Below, you’ll find real stories and clear explanations about how these changes happen, what the FDA does (and doesn’t) tell you, and how to protect yourself when your medication doesn’t feel the same. You’re not imagining things. And you don’t have to guess what’s going on.
Learn what manufacturing changes trigger FDA re-evaluation for generic drugs, how the approval system works, and how new programs are cutting review times. Essential for manufacturers, pharmacists, and healthcare professionals.