Every time you pick up a prescription and see a lower price tag than the brand-name version, you’re seeing the result of the Abbreviated New Drug Application (ANDA) process. This isn’t just paperwork - it’s the backbone of affordable medicine in the U.S. The ANDA lets generic drugmakers get their products on the market without repeating every single clinical trial ever done on the original drug. It’s how 90% of prescriptions in America end up being generics - saving patients and the healthcare system billions every year.
What Exactly Is an ANDA?
The ANDA is a regulatory shortcut created by the Hatch-Waxman Act in 1984. Before this law, generic drugmakers had to prove a drug was safe and effective from scratch - just like the original brand. That meant spending hundreds of millions and waiting years. The ANDA changed that. Instead of redoing all the clinical studies, a generic company only needs to prove their version works the same way as the brand-name drug - called the Reference Listed Drug (RLD).
To qualify, the generic must match the RLD in four key ways:
- Same active ingredient
- Same strength and dosage form (pill, injection, cream, etc.)
- Same route of administration (taken by mouth, injected, inhaled)
- Same conditions of use (what it treats, who can take it)
Labeling has to be nearly identical too, with only minor exceptions allowed. And the manufacturing must follow current Good Manufacturing Practices (cGMP). That’s it. No new clinical trials on humans. No repeating animal studies. Just solid proof that the generic behaves the same in the body.
How Bioequivalence Proves a Generic Works
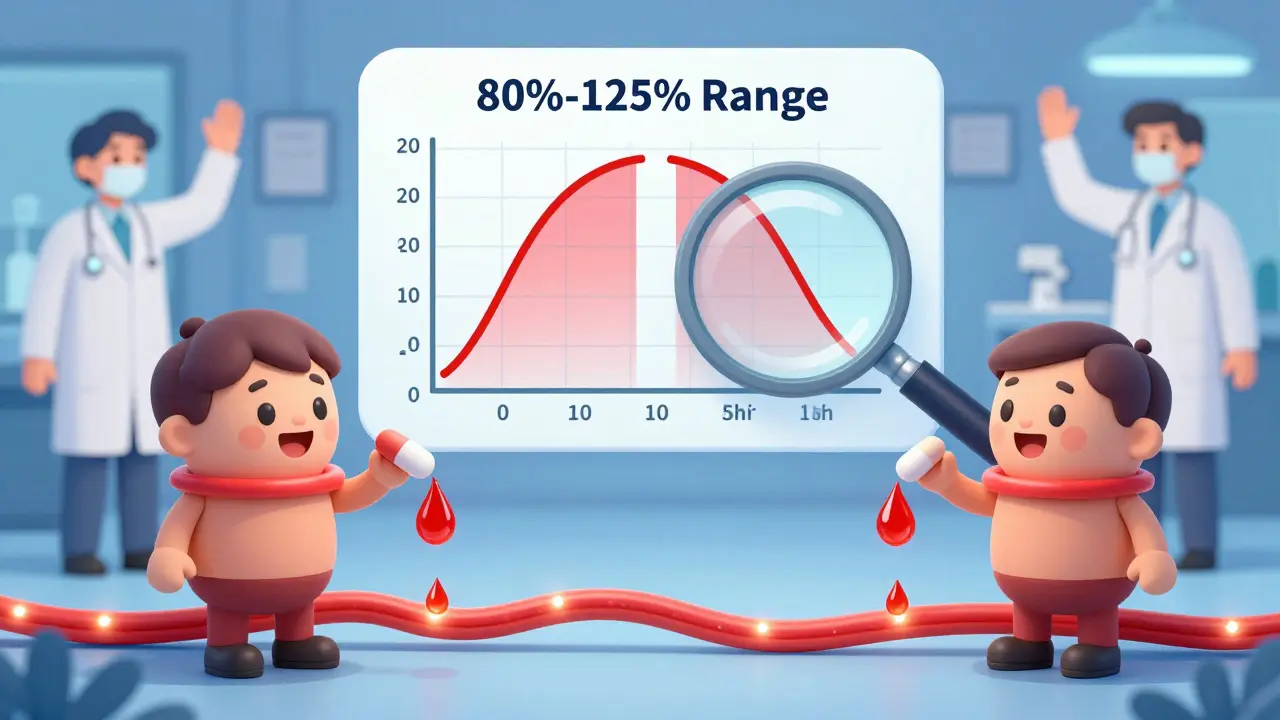
The real magic of the ANDA is bioequivalence. This isn’t just about looking the same or tasting the same. It’s about proving the generic drug enters the bloodstream at the same rate and to the same extent as the brand. If the brand releases 100 mg of medicine into your blood over 4 hours, the generic must do the same.
Manufacturers run small, controlled studies - usually with 24 to 36 healthy volunteers. They give half the group the brand, half the generic, then measure blood levels over time. If the generic’s levels fall within 80% to 125% of the brand’s, it’s considered bioequivalent. That’s the FDA’s standard. It’s not guesswork. It’s science with strict thresholds.
But it’s not always simple. For complex products - like inhalers, injectables, or topical creams - bioequivalence gets harder. A cream might look identical, but if the active ingredient doesn’t penetrate the skin the same way, it won’t work the same. That’s why 35% of Complete Response Letters from the FDA in 2022 cited problems with bioequivalence studies. Some companies spend over $1 million and go through three rounds of testing just to get it right.
The ANDA Submission Process: Four Phases
Getting an ANDA approved isn’t a one-step process. It’s a four-phase journey that can take years:
- Submission and Filing Review - The FDA checks if your application has all the required forms, data, and fees. If something’s missing, they’ll reject it outright. This takes up to 60 days.
- Discipline Reviews - Teams of scientists review different parts: chemistry, manufacturing, labeling, microbiology, and bioequivalence. Each team looks for gaps or inconsistencies. This is where most problems show up.
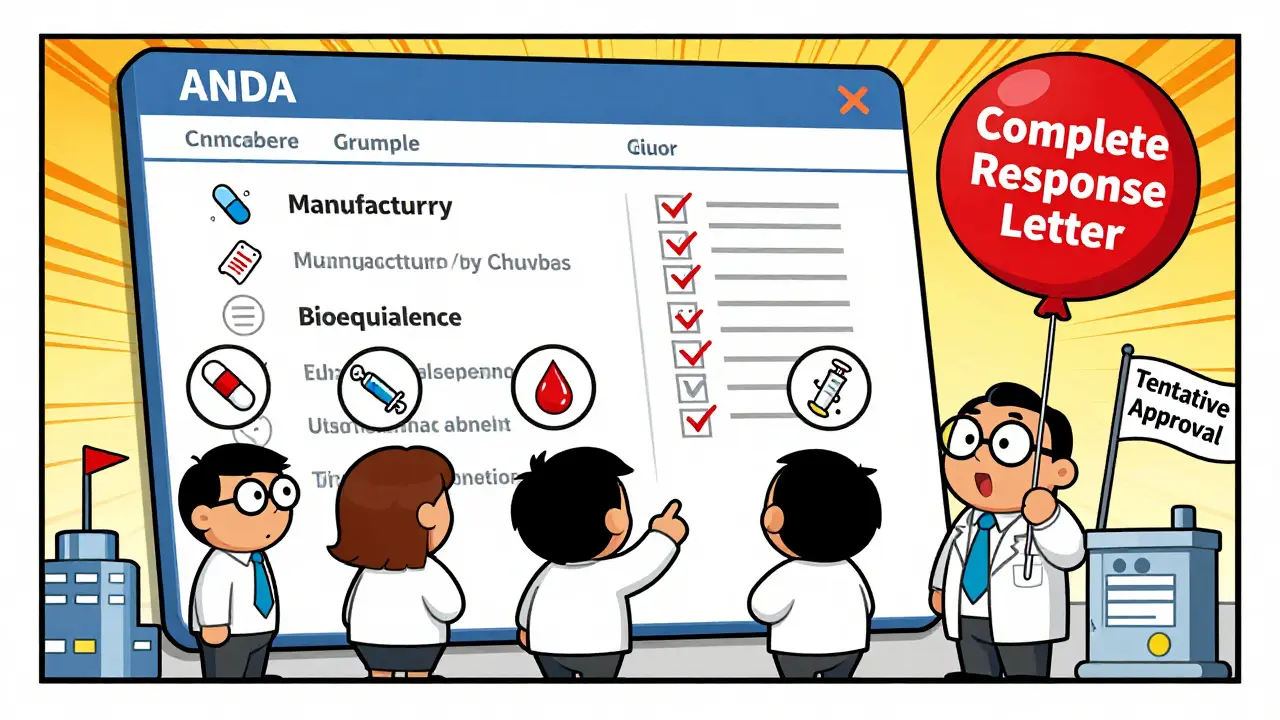
- Information Requests and Responses - If a team finds an issue, they send an Information Request (IR). You have to answer - often with new data or revised documents. Most applicants get 10 to 20 IRs. Some get a Complete Response Letter (CRL), which means the application can’t be approved as-is.
- Final Approval or Tentative Approval - If everything checks out and there are no patent or exclusivity blocks, you get Final Approval. If there’s a patent still in effect, you get Tentative Approval. You can’t sell the drug yet, but you’re ready to go the moment the patent expires.
Under the Generic Drug User Fee Amendments (GDUFA III), the FDA aims to complete its review of original ANDAs within 10 months. But in reality, the average time from submission to approval is around 30 months. Why? Because of delays in inspections, back-and-forth on IRs, and complex products.
Why Generic Drugs Are So Much Cheaper
Brand-name drugs cost $2.3 billion on average to develop - including failed attempts, clinical trials, and marketing. Generic companies spend between $1 million and $5 million per ANDA. That’s a 99% cost reduction.
That difference shows up in your pharmacy. Within a year of a generic hitting the market, prices drop to about 15% of the brand’s cost. In 2021 alone, generics saved the U.S. healthcare system $373 billion. That’s not just a number. That’s a diabetic patient who can afford insulin. A heart patient who doesn’t skip doses because of cost. A senior on a fixed income who can still get their blood pressure meds.
And it’s not just about price. It’s about access. Without the ANDA process, millions of Americans couldn’t afford the drugs they need. The system works because it doesn’t reinvent the wheel - it builds on what’s already proven.
Common Reasons ANDAs Get Rejected
Even with clear rules, many ANDAs fail. The most common reasons:
- Bioequivalence issues - 35% of CRLs. Studies don’t meet FDA standards. Sample sizes too small. Statistical analysis flawed.
- Manufacturing problems - 28%. Facilities don’t meet cGMP. Documentation is sloppy. Process controls aren’t tight enough.
- Labeling errors - 22%. Warnings missing. Dosage instructions unclear. Contradicts the brand’s label.
- Patent or exclusivity blocks - Often not a scientific issue, but a legal one. If the brand still has patent protection, the FDA can’t approve the generic yet.
One manufacturer shared that their first ANDA received 17 Information Requests over 14 months. Another spent $1.2 million on three rounds of bioequivalence testing for a topical cream. These aren’t rare stories. They’re the norm.
Who’s Winning in the Generic Market?
The U.S. generic market is worth $127.6 billion and growing. The biggest players are Teva Pharmaceuticals (22% market share), Viatris (15%), and Sandoz (12%). But 75% of ANDAs come from companies that already have five or more approved generics. Experience matters.
Companies that succeed long-term don’t just file applications. They build systems. They use Quality by Design (QbD) principles to make manufacturing more predictable. They hold pre-ANDA meetings with the FDA to iron out issues before submitting. They have teams of 10 to 15 people working full-time on one application - that’s 5,000 to 10,000 hours of work per submission.
And the FDA helps. They’ve published over 2,000 product-specific guidances - detailed instructions for how to approve each type of drug. In 2022 alone, they added 450 new ones. If you’re making a generic inhaler, there’s a guide for that. If you’re making a patch, there’s a guide for that too.
What’s Next for the ANDA Process?
The FDA is shifting focus to complex generics - things like nasal sprays, injectables, and topical products that are harder to copy. These now make up 35% of pending ANDAs. The agency is using AI to speed up chemistry reviews. Over 78% of FDA reviewers now use AI tools to check data faster and more accurately.
Real-world evidence is also becoming more important. Instead of just lab studies, regulators are looking at how generics perform in real clinics. And international standards are aligning through the International Council for Harmonisation (ICH), making it easier for global manufacturers to meet U.S. requirements.
But challenges remain. Patent thickets - where brand companies stack multiple patents to delay generics - still block access. Risk Evaluation and Mitigation Strategies (REMS) can make it hard for generic makers to get samples of the brand drug for testing. These aren’t scientific problems. They’re legal and policy ones.
Still, the ANDA process has delivered. It’s the reason you can buy a month’s supply of metformin for $4. It’s why asthma inhalers are affordable. It’s why millions of people don’t have to choose between medicine and rent.
What You Should Know as a Patient
When your doctor prescribes a brand-name drug, ask: "Is there a generic?" The answer is almost always yes. The FDA considers generics just as safe and effective as the brand. They’re not cheaper because they’re worse - they’re cheaper because they don’t need to repeat expensive research.
Switching from brand to generic is safe. The FDA monitors adverse events for both. If a generic causes a problem, it’s investigated like any other drug. No exceptions.
And if your pharmacy tries to switch you to a different generic? That’s fine too. Multiple generics can exist for the same brand. Each one meets the same standards. They’re all approved. The only difference might be the shape, color, or inactive ingredients - none of which affect how the drug works.
The ANDA process isn’t perfect. But it’s the most successful system in the world for making life-saving medicines affordable. And it’s working - every day, for millions of people.
Are generic drugs as safe as brand-name drugs?
Yes. The FDA requires generic drugs to meet the same strict standards for quality, strength, purity, and stability as brand-name drugs. They must be bioequivalent - meaning they work the same way in the body. The FDA monitors adverse events for both types of drugs equally. There is no difference in safety.
Why does my generic look different from the brand?
By law, generic drugs can’t look exactly like the brand because of trademark rules. That’s why the shape, color, or markings might be different. But the active ingredient, dosage, and how it works in your body are identical. The differences are only in inactive ingredients like dyes or fillers, which don’t affect the drug’s effectiveness.
Can I trust a generic drug if it’s made overseas?
Yes. The FDA inspects all manufacturing facilities - whether in the U.S., India, China, or elsewhere - to ensure they meet current Good Manufacturing Practices (cGMP). Over half of generic drugs sold in the U.S. are made overseas, and every facility is subject to the same inspections as U.S.-based ones. The FDA doesn’t allow imports from facilities that fail inspections.
What does Tentative Approval mean?
Tentative Approval means the FDA has found your generic drug scientifically acceptable, but it can’t be sold yet because of patent or exclusivity protections on the brand-name drug. Once those protections expire, your drug can be fully approved and marketed immediately - no further review needed. It’s like being on the waiting list with priority status.
How long does it take to get an ANDA approved?
The FDA aims to complete its review within 10 months under GDUFA III. But in practice, most ANDAs take 24 to 30 months from submission to approval. Delays often come from Information Requests, facility inspections, or complex products. Some generics for critical shortages get expedited review and can be approved in under 12 months.









laura Drever
generic drugs r fine i guess but why do they always taste like chalk??
Diana Campos Ortiz
I’ve been on generic metformin for 7 years and never had an issue. My grandma switches between 3 different brands every month and still keeps her A1c stable. The FDA’s system actually works.
People act like generics are some shady loophole, but they’re just smart science. No need to re-invent the wheel when the original already passed every test.
Jesse Ibarra
Oh great. So now we’re glorifying corporate shortcuts as ‘innovation’? The FDA lets companies skip clinical trials and calls it ‘efficiency.’ Meanwhile, real innovation is getting crushed by patent trolls and Big Pharma’s lobbying machine.
This isn’t progress - it’s regulatory capture dressed up as a public service. You think $4 metformin is a win? Wait till the next batch gets contaminated because the inspection was done over Zoom by someone who doesn’t speak Mandarin.
James Castner
It’s worth pausing to recognize the profound philosophical shift embodied by the ANDA process: the recognition that knowledge is cumulative, not proprietary. The original innovator bore the burden of discovery - the moral and financial risk - and society owes them that. But once that knowledge is validated, to demand its re-creation for every subsequent practitioner is not fidelity to science - it is intellectual hoarding.
The ANDA is not a loophole; it is the ethical crystallization of scientific progress. We do not require every carpenter to invent the hammer. We do not require every physician to rediscover the circulatory system. Why, then, should every generic manufacturer be forced to re-verify what has already been empirically established?
The real tragedy isn’t the approval of generics - it’s that we still treat medicine as a commodity to be locked behind patents, rather than a public good to be democratized through shared knowledge. The system works. But it could work better - if we stopped celebrating the shortcut and started demanding the removal of the gatekeepers.
Adam Rivera
Just wanted to say thanks for writing this. I’m from a small town in Alabama where people still think generics are ‘fake meds.’ I shared this with my mom and she finally stopped refusing her blood pressure pills because ‘they look different.’
It’s small stuff, but it matters. You made someone’s life easier today.
Rosalee Vanness
Let’s talk about the quiet heroes behind this system - the regulatory scientists who spend 14 hours a day poring over chromatography graphs and manufacturing logs. They’re not in the headlines. They don’t get LinkedIn posts. But they’re the reason your insulin doesn’t kill you because someone cut corners on the fill volume.
I used to work in pharma QA. I’ve seen the spreadsheets. I’ve seen the labs. I’ve seen the sleepless nights when a bioequivalence study failed on the 18th volunteer because the lab fridge spiked 2 degrees for 47 minutes.
These aren’t just forms. They’re lifelines. And the FDA’s reviewers? They’re not bureaucrats. They’re the last line of defense between a patient and a faulty batch.
So next time you grab that $4 pill, don’t just thank the system. Thank the person who stayed late to verify that the dissolution profile matched within 95% confidence. That’s the real magic.
lucy cooke
Oh wow. Another whitepaper masquerading as a public service announcement. How poetic. We’re told to trust the FDA while the same agency approves generics from factories that burn coal to power their ovens.
It’s not about science - it’s about spectacle. The FDA gives a stamp of approval like a carnival barker saying ‘this is the real thing!’ while the real thing is being manufactured in a basement in Hyderabad with a 2008 HPLC machine and a guy who learned English from a YouTube video.
And we’re supposed to feel safe? Please. This isn’t healthcare. It’s global capitalism with a lab coat.
Trevor Davis
My cousin works at a generic lab in Indiana. He says the hardest part isn’t the science - it’s the paperwork. One typo in the batch number and the whole thing gets bounced back.
And yeah, some companies cut corners. But most? They’re just trying to stay alive. Big Pharma’s got lawyers and lobbyists. These guys got Excel sheets and caffeine.
So next time you’re mad your generic looks different? Just remember - it’s not a knockoff. It’s a miracle made possible by people who don’t get paid enough to care about your opinion.
John Tran
People don’t get it. The ANDA isn’t about saving money - it’s about power. Who gets to decide what’s ‘bioequivalent’? The FDA? Or the corporations that fund the studies?
I’ve seen the data. Some bioequivalence studies are designed to fail - intentionally. Then the company resubmits with a tweaked formulation and gets approved. Rinse. Repeat.
And don’t get me started on the ‘pre-ANDA meetings.’ Those aren’t consultations. They’re covert negotiations where the FDA gives a company a roadmap to bypass their own rules.
This isn’t regulation. It’s collusion with a side of placebo.
mike swinchoski
Generic drugs are dangerous. They’re not tested enough. People die because they take cheap pills. The FDA is lazy and the government is corrupt. Stop lying to us.
Gregory Parschauer
Let’s be brutally honest: the ANDA system is a facade. The real bottleneck isn’t science - it’s the oligopoly of 4 manufacturers who control 80% of the market. The FDA doesn’t prevent monopolies. It enables them by granting exclusivity through technical loopholes.
Meanwhile, the real innovation is happening in biosimilars and AI-driven formulation design - but those are blocked by patent thickets and REMS abuse.
And let’s not pretend the ‘$4 metformin’ is a win. It’s a symptom of a broken system where the only thing cheaper than the drug is the patient’s dignity.
We don’t need more ANDAs. We need a healthcare system that treats medicine as a human right - not a commodity to be auctioned off to the lowest bidder with the best regulatory lawyers.