When you pick up a prescription, you might see two options: the brand-name pill you recognize from TV ads, or a much cheaper generic version with a different color and shape. You might wonder - does the generic really work the same? The answer isn’t just yes - it’s backed by one of the most rigorous drug approval systems in the world, run by the U.S. Food and Drug Administration (FDA).
Why Generic Drugs Are Allowed to Skip Big Clinical Trials
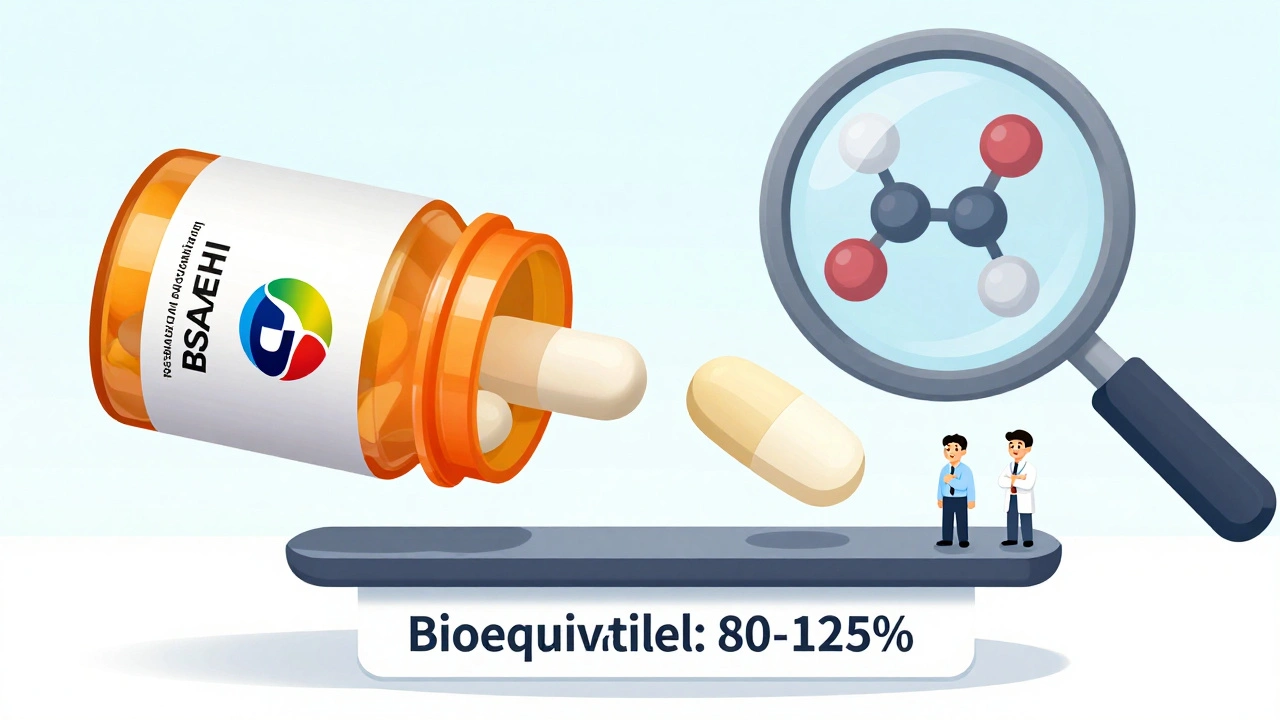
Generic drug makers don’t have to repeat the expensive, years-long clinical trials that brand-name companies did. That’s not because they’re cutting corners - it’s because the FDA already knows the original drug works. The legal foundation for this is the Hatch-Waxman Act of 1984. This law created a shortcut called the Abbreviated New Drug Application (ANDA). It lets generic manufacturers prove their product is equivalent without starting from scratch.The FDA doesn’t assume equivalence. It demands proof. That proof comes from bioequivalence studies - tightly controlled tests in healthy volunteers. These studies measure how much of the active drug enters the bloodstream and how fast. For a generic to be approved, its absorption profile must fall within 80% to 125% of the brand-name drug’s. This isn’t a rough estimate. It’s a statistical requirement based on the 90% confidence interval for two key measurements: AUC (total drug exposure) and Cmax (peak concentration).
These studies usually involve just 24 to 36 people. But they’re not simple. They’re conducted in fasting and sometimes fed states, using the same analytical methods as the original drug. The FDA publishes specific bioequivalence requirements for over 1,500 drugs in its public database. If your drug is on that list, the manufacturer must follow exact study rules - down to the number of blood samples and the timing.
It’s Not Just About the Active Ingredient
Many people think generics are just the same active ingredient in a cheaper bottle. That’s only part of the story. The FDA requires that the generic has the same active ingredient, strength, dosage form (tablet, capsule, liquid), and route of administration (oral, injectable, etc.) as the brand. But inactive ingredients - the fillers, dyes, and binders - can be different.That’s okay, as long as they’re safe. The FDA maintains an Inactive Ingredient Database that lists acceptable amounts of over 500 excipients across 80 delivery methods. If a generic uses a new excipient or a higher dose than what’s on file, the manufacturer must prove it doesn’t affect absorption or safety. For example, a generic version of a slow-release tablet might use a different polymer to control release, but it must still deliver the drug at the same rate as the brand.
Manufacturing is held to the same standard too. Every facility - whether it’s in the U.S., India, or Germany - must follow Current Good Manufacturing Practices (cGMP). The FDA inspects these sites regularly. In 2022, 21% of rejections for generic approvals were due to manufacturing issues, like inconsistent tablet hardness or contamination risks. The FDA doesn’t care if you’re a big company or a small startup. If your pills don’t meet USP <905> standards for content uniformity (85% to 115% of labeled strength), they won’t be approved.
The ANDA Approval Process Is a Gauntlet
Getting a generic approved isn’t easy. It’s not a formality. It’s a multi-year, multi-million-dollar process. A typical ANDA costs between $1.5 million and $3 million and takes 3 to 5 years from start to finish. For complex products like inhalers or topical creams, costs can hit $25 million.The application itself is massive - 30,000 to 50,000 pages of data, organized in a format called the Common Technical Document. The bioequivalence section alone can be 5,000 to 10,000 pages of raw data, statistical models, and study reports. The FDA reviews every page.
Here’s how it breaks down:
- Filing Review (60 days): The FDA checks if the application is complete. In 2022, 35% of submissions were refused outright for missing key data or poor organization.
- Substantive Review: Teams of chemists, pharmacologists, and statisticians examine chemistry, manufacturing, bioequivalence, and labeling. Each discipline has to sign off.
- Inspection: The FDA inspects the manufacturing site. If the site has never been inspected or has a history of violations, it’s a red flag.
- Decision: The FDA either approves or issues a Complete Response Letter (CRL). In 2022, the most common reasons for rejection were inadequate bioequivalence studies (28%), manufacturing issues (22%), and labeling errors (18%).
Even after approval, the FDA keeps watching. The agency can pull a generic off the market if post-market data shows problems. In 2023, over 14,000 generic drugs were approved and on the market. That’s not luck. It’s the result of a system designed to catch errors before patients ever see the pill.

Are There Any Exceptions? What About Narrow Therapeutic Index Drugs?
Most generics are safe to swap. But there’s a small group of drugs where tiny differences in blood levels can cause serious harm. These are called narrow therapeutic index (NTI) drugs - things like warfarin, levothyroxine, phenytoin, and cyclosporine.For these, the FDA applies tighter standards. Instead of 80-125%, the acceptable range for AUC and Cmax is 90-111%. That’s a much narrower window. The agency also requires additional testing, like multiple-dose studies, to make sure the drug behaves consistently over time.
Even with these extra steps, some doctors remain cautious. A 2020 study in JAMA Internal Medicine found that 11% of physicians still worry about switching patients on NTI drugs to generics. The FDA acknowledges this concern and maintains a public list of NTI drugs requiring special bioequivalence criteria. But real-world data tells a different story. A 2023 analysis of 15 million patient records found no difference in outcomes between brand and generic versions of NTI drugs like levothyroxine.
What Do Patients and Pharmacists Really See?
Patients sometimes report feeling different on a generic. They might say, “This one doesn’t work as well,” or “I feel more tired.” These reports are real - but they’re rarely backed by science.Pharmacists, who see these switches every day, mostly don’t see a difference. A 2022 Reddit thread with 147 pharmacist comments showed 82% reported no meaningful clinical differences. The 18% who did noted issues mostly with complex delivery systems - like inhalers or topical creams - where small formulation changes might affect how the drug is absorbed through skin or lungs.
Adverse event reports tell a similar story. The FDA’s FAERS database shows generic drugs have the same rate of reported side effects per million prescriptions as brand-name drugs - 1.7 vs. 1.6. That’s statistically identical.
But perception matters. A 2021 survey found 37% of independent pharmacists said patients expressed concerns about generics. The most common concerns came from people on long-term meds for neurological or psychiatric conditions. The FDA’s own 2020 patient survey found that while 89% trusted FDA-approved generics, 41% had switched between brand and generic at least once - and 12% thought they felt a difference. Yet when tested objectively, those perceived differences rarely held up in clinical measurements.

Why Generic Drugs Save Billions - And How That Changes Healthcare
Generic drugs aren’t just cheaper. They’re transformative. In 2023, generics made up 90% of all prescriptions filled in the U.S. But they cost only 23% of what brand-name drugs do. That’s a $313 billion annual savings for the American healthcare system, according to the Association for Accessible Medicines.That savings doesn’t just help insurance companies. It helps patients. A 2023 IQVIA study found that adherence rates - how consistently people take their meds - were 3.2% higher for generics than brands. Why? Because lower copays mean people don’t skip doses or split pills.
That’s why the FDA is pushing harder than ever to get more generics approved. The 2022 Generic Drug User Fee Amendments (GDUFA III) set a goal of cutting review times to 8 months for standard applications and 6 months for priority ones. In 2023, the FDA approved 1,023 generic drugs - including 107 that were the first-ever versions of their brand-name counterparts. That’s up 15.7% from the year before.
Big companies like Teva, Viatris, and Sandoz dominate the market, but over half of all approvals go to smaller manufacturers. The FDA’s Generic Drug Competition Action Plan is designed to break up monopolies and prevent shortages. For example, when a drug like insulin or epinephrine runs short, the FDA fast-tracks generic applications - sometimes approving them in 6 months instead of 10.
What’s Next? The Future of Generic Drugs
The next frontier is biosimilars - generic versions of complex biologic drugs like Humira, Enbrel, and Keytruda. These aren’t small molecules like aspirin or metformin. They’re made from living cells. The FDA is preparing to launch a dedicated pathway for biosimilars by 2025, with 15 new product-specific guidances coming in early 2024.Also on the horizon: more generics for cancer drugs. The FDA’s Real-Time Oncology Review pilot, expanded in 2023 to include generics, approved the first generic capecitabine in February 2024 - seven months faster than normal. That’s life-saving speed.
By 2028, over $260 billion in brand-name drug revenue will lose patent protection. That’s a tidal wave of new generic opportunities. The FDA’s job isn’t just to approve them - it’s to make sure every single one works exactly as well as the brand. And so far, the system has delivered.
Are generic drugs as effective as brand-name drugs?
Yes. The FDA requires generic drugs to prove they are bioequivalent to the brand-name version - meaning they deliver the same amount of active ingredient into the bloodstream at the same rate. This is tested in clinical studies with healthy volunteers and must meet strict statistical standards. Over 90% of prescriptions filled in the U.S. are generics, and decades of real-world data show no meaningful difference in effectiveness or safety for the vast majority of drugs.
Why do generic drugs look different from brand-name drugs?
By law, generic drugs can’t look exactly like the brand-name version - that would be trademark infringement. So they may have different colors, shapes, or markings. But the active ingredient, strength, dosage form, and how it works in the body are identical. The differences in appearance come from different inactive ingredients, like dyes or fillers, which are chosen to meet safety standards but don’t affect how the drug works.
Can I trust generics made outside the U.S.?
Yes. The FDA inspects all manufacturing facilities - whether they’re in the U.S., India, China, or elsewhere - before approving a generic drug. In fact, over 80% of generic drugs sold in the U.S. are made overseas. The FDA uses the same quality standards for all sites. If a foreign plant fails inspection, the FDA can block imports and refuse approval until problems are fixed.
Are there any drugs I should avoid switching to generics?
For most drugs, switching is safe. But for a small group called narrow therapeutic index (NTI) drugs - like warfarin, levothyroxine, or phenytoin - even small changes in blood levels can cause problems. The FDA requires stricter bioequivalence testing for these, and many doctors still prefer to stick with one version. If you’re on one of these drugs and feel different after switching, talk to your doctor. But don’t assume the generic is faulty - the difference is often psychological or due to other factors.
Why are generic drugs so much cheaper?
Generic drugs are cheaper because manufacturers don’t have to repeat the expensive clinical trials that brand-name companies did. They also don’t spend millions on advertising. The FDA’s approval process is faster and less costly for generics, and competition among multiple manufacturers drives prices down. In 2023, generics saved the U.S. healthcare system $313 billion - money that goes directly into lower out-of-pocket costs for patients.








Rebecca Braatz
So many people still think generics are 'cheap knockoffs'-like they're just rebranded aspirin from a gas station. The FDA doesn't cut corners. They make sure every pill, every batch, every excipient is held to the same standard as the brand. I work in pharmacy and I've seen the paperwork. It's insane how much data they review. If you're worried about your meds, trust the system-not your cousin's Reddit post.
Pavan Kankala
Yeah right. The FDA's just a puppet for Big Pharma. They approve generics from India and China because they're paid off. You think those labs are clean? I've seen videos-flies in the vats, workers not wearing gloves. And don't get me started on the 'bioequivalence' numbers. 80-125%? That's a 45% swing! My uncle took generic warfarin and ended up in the ER. Coincidence? I think not.
Yasmine Hajar
Y'all are overthinking this. I've been on generic levothyroxine for 8 years. Same dose. Same results. My TSH hasn't budged. I switched from Synthroid because my insurance wouldn't cover it-and I didn't feel any different. My mom switched to generic metoprolol after her heart surgery and she's hiking mountains now. If you're scared, talk to your pharmacist. They see hundreds of switches a week. They know what's real.
Also-yes, generics are made overseas. So are your phone, your shoes, your coffee. The FDA inspects those factories. If they didn't, we'd have a whole lot more recalls. Not every foreign factory is a dump. Some are cleaner than your kitchen.
Ashley Elliott
Interesting. I appreciate the detail. I’ve always trusted generics, but I never knew about the 85-115% content uniformity rule. Or that they test in fasting AND fed states. That’s actually really reassuring. I’ve had friends panic over switching, but I think most of it’s just the placebo effect-like when you think your new phone battery drains faster just because it’s a different brand.
Also, the fact that the FDA publishes exact requirements for over 1,500 drugs? That’s transparency. Most people don’t even know that exists.
Chad Handy
Let me tell you about the time I took a generic version of my antidepressant because my insurance forced me to. I felt like a zombie for two weeks. My hands shook. I couldn’t focus. I went back to the brand and everything snapped back. The FDA says it’s all in my head. But my body knew the difference. And now I pay out of pocket-$200 a month-because I’m not risking my mental health for a $10 savings. You don’t know what it’s like until you’re the one losing sleep because your meds don’t work.
And don’t even get me started on the '36 healthy volunteers' study. That’s not science. That’s a guess. What about people with liver disease? Diabetics? Elderly? They never test on us. They test on college kids who drink protein shakes and do yoga. That’s not real life.
Augusta Barlow
They’re lying. The FDA doesn’t inspect every factory. They send a guy for one day. He gets a tour. They clean up the night before. The real truth? The FDA has a backlog of 1,000+ applications. They approve them just to look like they’re doing something. That’s why we have shortages. That’s why people are dying. And you know who benefits? The big pharma companies-they buy up the generics, then raise the price. It’s a monopoly disguised as competition.
I read the GDUFA III documents. They’re written in legalese so thick you need a PhD to understand it. That’s not oversight. That’s obfuscation. And don’t tell me about 'real-world data'-data can be manipulated. I’ve seen the spreadsheets. They cherry-pick the good outcomes. The bad ones? Buried in Appendix C.
Jenny Rogers
It is imperative to underscore that the regulatory framework governing generic pharmaceuticals in the United States is not merely a procedural formality; it constitutes a rigorous, science-based, and legally codified mechanism for ensuring therapeutic equivalence. The Hatch-Waxman Act, as codified under Title 21 of the U.S. Code, was a landmark legislative achievement that harmonized innovation with accessibility. The statistical parameters governing bioequivalence-specifically, the 90% confidence interval for AUC and Cmax-were derived from robust pharmacokinetic modeling and peer-reviewed methodology. To assert that these standards are insufficient is to fundamentally misunderstand the nature of pharmaceutical science.
Furthermore, the assertion that foreign manufacturing facilities are inherently inferior is not only factually incorrect but also ethically indefensible. The FDA maintains a global inspectional presence, and over 80% of generic drugs are manufactured under cGMP conditions that are, in many cases, superior to those found in domestic facilities.
Rachel Bonaparte
Okay, but let’s be real-this whole system is a scam. The FDA lets generics in, but they’re still making billions off the brand-name versions. And don’t tell me about 'competition'-when there are only three manufacturers for a drug, it’s not competition, it’s a cartel. I’ve seen the price of generic metformin go from $4 to $120 in five years. Why? Because the other companies got bought out and now it’s just one company making it. And the FDA? They just nod along. They’re not protecting patients-they’re protecting the system.
Also, the fact that they let you use different dyes? That’s wild. What if you’re allergic to Red 40? You don’t even know what’s in your pill. They don’t have to tell you. That’s not transparency. That’s negligence.
Michael Feldstein
Biggest myth: 'Generics are just as good.' Not always. For NTI drugs like warfarin or levothyroxine, the 90-111% window is critical-but even then, formulation differences can matter. The FDA’s standards are solid, but they’re not magic. If you’re on a high-risk med, stick with the brand if you can. Or, better yet, ask your pharmacist for the same manufacturer every time. Consistency matters more than you think.
Also, the '36 healthy volunteers' thing? That’s the part most people miss. Those studies are done on young, healthy people. But most patients on these drugs are older, with kidney issues, liver disease, or on 5 other meds. That’s not accounted for in the approval process. So yeah, the system works on paper. But real life? It’s messier.
jagdish kumar
Life is a cycle. The pill is a symbol. The FDA? Just another wheel in the machine.
zac grant
Let’s talk about the real win here: adherence. A 3.2% increase in adherence with generics? That’s not just a stat-that’s thousands of lives saved. People skip meds because they’re expensive. Generic insulin means a diabetic doesn’t have to choose between food and medicine. That’s not corporate altruism-that’s public health policy working. The FDA’s job isn’t to make drugs cheap-it’s to make them accessible. And they’re succeeding.
Also, biosimilars are coming. That’s the next frontier. Biologics cost $100k/year. If we can get biosimilars approved fast and safe, we’re talking about cutting cancer treatment costs by 80%. This isn’t just about pills-it’s about equity.
michael booth
Generic drugs are a triumph of regulatory science. The ANDA pathway reduces duplication of clinical trials without compromising safety. The bioequivalence standards are statistically rigorous and empirically validated. The FDA’s inspection protocols are transparent and globally harmonized. The economic impact-$313 billion in savings-is not incidental; it is systemic and essential to the sustainability of American healthcare.
Perceived differences in patient experience are often attributable to psychosocial factors, including branding bias and confirmation bias. The data does not support clinical inferiority. The system works.
Libby Rees
Generics work. I’ve been on them for 15 years.
Rebecca Braatz
Chad, I get it. You had a bad experience. But your story doesn’t invalidate the data. The FDA gets thousands of adverse reports every year. Most are unrelated to the drug. If your antidepressant made you feel like a zombie, it could’ve been the switch, the timing, or even stress. But 99% of people don’t have that problem. That’s why they don’t pull generics off the market. Because the system catches the rare bad batches before they reach you.