Spotting a fake pill can be terrifying. You take it expecting relief, but instead, you get no effect-or worse, you get sick. Counterfeit drugs don’t just fail to work. They can contain toxic chemicals, wrong doses, or no active ingredient at all. In 2022, the global market for fake medicines hit $231.6 billion, with online pharmacies selling most of them. And while the U.S. has strong systems to catch these, they only work if people report them.
Why reporting fake drugs matters
Every time you report a suspicious pill, you help shut down a criminal operation. The FDA received over 100,000 adverse event reports in 2022, and counterfeit drug reports are rising fast. Since the pandemic, online drug sales have exploded-96% of online pharmacies checked in 2022 were illegal. These sites sell fake versions of everything from Adderall to insulin to heart meds.
But here’s the thing: most people don’t report. They throw the pill away, assume it was a bad batch, or worry they’ll get in trouble. That’s exactly what the criminals count on. When you report, you give authorities the clues they need to trace the supply chain, seize shipments, and arrest traffickers. In 2022, the FDA’s Office of Criminal Investigations closed 1,842 counterfeit drug cases, leading to 187 criminal convictions. None of those would’ve happened without reports from people like you.
What counts as a counterfeit drug?
Not every weird-looking pill is fake-but many are. Here’s what to look for:
- Spelling errors on the label or packaging (78% of counterfeits have them)
- Missing or fake lot numbers (63% of fakes lack valid ones)
- Wrong color, shape, or texture compared to what you normally get (52% of cases)
- Packaging that looks cheap-faded ink, loose seals, mismatched fonts (87% of fakes have this)
- Unfamiliar brand names or a pharmacy you’ve never heard of
If your prescription suddenly tastes different, doesn’t work like it used to, or causes new side effects, don’t ignore it. Keep the pill and its packaging. Don’t throw it away. That’s your evidence.
How to report in the U.S.
The U.S. has two main ways to report fake drugs-choose based on what you know and how urgent it is.
1. Use FDA MedWatch (for consumers)
This is your go-to if you took a suspicious pill and had a bad reaction-or even if you just think it’s fake. You don’t need proof. You just need details.
Go to www.fda.gov/medwatch and fill out Form 3500 online. You’ll need:
- Drug name and strength (e.g., “Metformin 500 mg”)
- Lot number (found on the bottle or box)
- National Drug Code (NDC) - a 10-digit number on the packaging
- Where you bought it (pharmacy name, website, street vendor)
- Any symptoms you had after taking it
- Photos of the packaging and pill (highly recommended)
Electronic submissions get a confirmation email within 72 hours. Paper forms take up to two weeks. The FDA says reports with photos are processed 89% faster. If you’re unsure what to write, call the FDA Drug Information Line at 855-543-3784. Average wait time: under 4 minutes.
2. Report criminal activity to FDA OCI
If you suspect a large-scale operation-like someone selling fake drugs from a website, social media account, or warehouse-use the FDA’s Office of Criminal Investigations (OCI) portal at www.fda.gov/oci. This is for serious cases: organized sellers, bulk shipments, or if you’re a pharmacist or pharmacy worker.
OCI requires more detail: dates, locations, suspect descriptions, and proof you’ve preserved evidence. They respond to high-priority reports within 48 hours. In 2022, they opened 1,842 investigations and seized over 1.2 million fake pills at U.S. ports.
3. Call your pharmacy or manufacturer
If you got the drug from a local pharmacy, call them first. They’re required to report suspicious products to the FDA. Many manufacturers have direct lines too. Pfizer’s Global Security Operations Center responds within 4 business hours. Roche guarantees a reply within 24 hours. Even if they can’t act alone, they’ll pass your info to the FDA with the product’s full history.
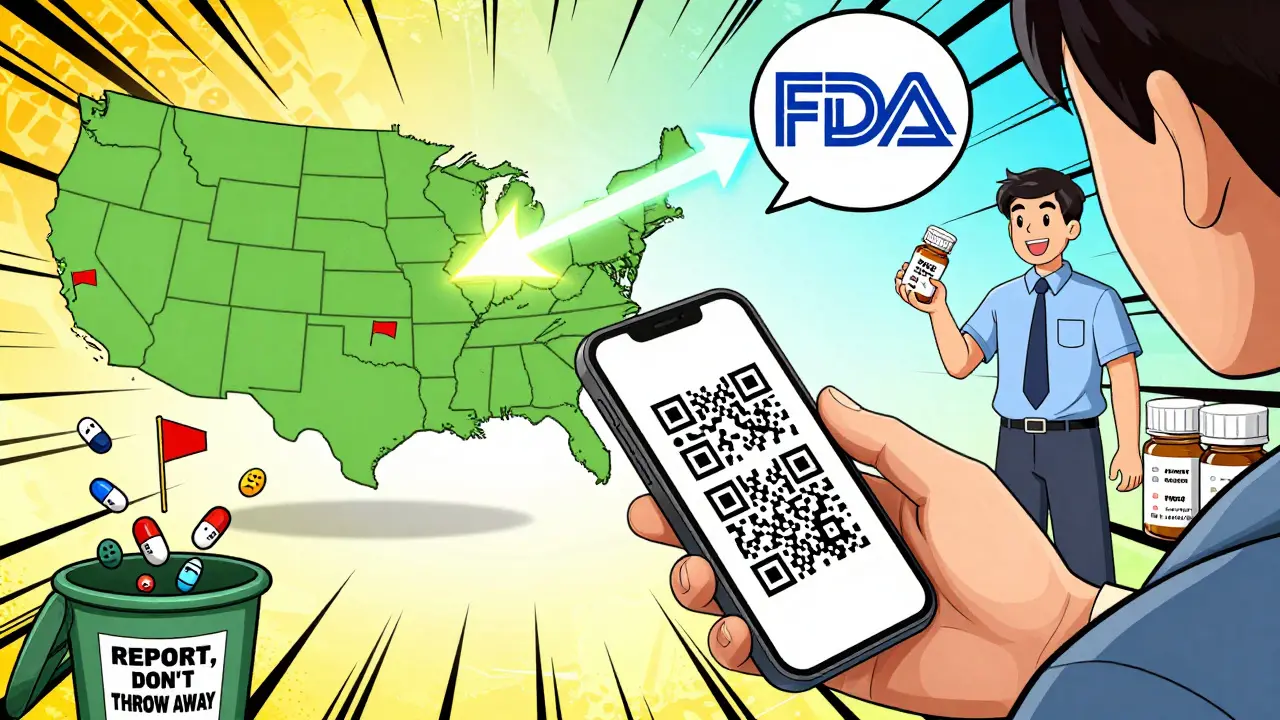
What if you’re outside the U.S.?
Counterfeit drugs are a global problem. The World Health Organization (WHO) runs a global reporting system for substandard and falsified medical products. Submit a report at who.int/falsifiedmeds. It’s available in 27 languages.
Another option is the Pharmaceutical Security Institute (PSI). They’ve tracked over 9,800 counterfeit incidents since 1991. You can email them at [email protected]. Their system uses AI to verify reports within 4.5 hours-faster than most government systems. But they require healthcare professionals to verify consumer reports, so if you’re not a clinician, stick with your country’s health authority.
In the EU, you report to your national medicines agency. In Canada, it’s Health Canada’s MedEffect program. In Australia, it’s the TGA. Always start with your country’s official health regulator.
What to do after you report
Don’t expect an immediate call back. The FDA says 63% of reporters don’t get updates on their case. That doesn’t mean nothing happened. It means the system is working behind the scenes.
Here’s what to do:
- Keep the packaging and pill. Don’t wash, destroy, or dispose of it.
- Write down when and where you bought it, and who sold it to you.
- If you took the drug and felt ill, see your doctor and tell them you suspect a counterfeit.
- Follow up after 10 business days if you haven’t heard anything. Call MedWatch at 1-800-FDA-1088.
One pharmacist in Ohio reported counterfeit insulin in 2022. She kept the original box with the lot number. The FDA traced it back to a warehouse in California within 12 hours. That single report led to the shutdown of a network selling 17,000 fake vials.
Common mistakes people make
Most reports fail because of simple errors:
- Not taking photos - text descriptions alone slow down investigations.
- Throwing away the packaging - the lot number is your best clue.
- Reporting to the wrong agency - 41% of people contact local police instead of the FDA.
- Waiting too long - the longer you wait, the harder it is to trace the product.
- Not following up - if you don’t ask, you won’t get answers.
And don’t assume your pharmacy is safe. A 2022 Consumer Reports survey found that 63% of people didn’t know where to report fake drugs. That confusion helps criminals stay hidden.

What’s changing in 2025?
Systems are getting smarter. The FDA launched a pilot program in 2023 that lets you scan a QR code on medicine packaging to report issues instantly. Over 20 drugmakers, including Pfizer and Merck, are on board.
By late 2024, the FDA plans to use blockchain to track every prescription drug from factory to pharmacy. In 2025, WHO will roll out a mobile app for reporting fake drugs in real time-just snap a photo and send it.
But none of this matters unless you use it. The most powerful tool in the fight against fake drugs isn’t technology. It’s you.
Frequently Asked Questions
What should I do if I think I took a counterfeit drug and feel sick?
Call your doctor or go to the emergency room immediately. Tell them you suspect a counterfeit drug. Then report it to the FDA through MedWatch. Don’t wait. Fake drugs can cause organ damage, allergic reactions, or even death. Keep the pill and packaging for evidence.
Can I report a fake drug I bought online?
Yes. Online pharmacies are the biggest source of counterfeit drugs. Report the website to the FDA’s OCI portal and also to the National Association of Boards of Pharmacy’s LegitScript program. Include screenshots of the site, order confirmation, and any communication with the seller.
Will I get in trouble for reporting a fake drug I bought without a prescription?
No. The FDA and other agencies focus on stopping the criminals, not punishing patients. Your report is confidential and protected. They care about the source of the drug, not how you got it. Reporting helps protect others.
How do I know if my pharmacy is legitimate?
Check if it’s licensed by your state pharmacy board. For online pharmacies, look for the VIPPS seal (Verified Internet Pharmacy Practice Sites) or use the National Association of Boards of Pharmacy’s website to verify the site. If it doesn’t require a prescription or offers drugs at 80% off, it’s likely fake.
Do I need to know the exact drug name to report?
No. If you’re unsure, describe the pill: color, shape, markings, packaging. Include any text on the pill or bottle. The FDA can often identify the drug from photos and descriptions. Better to report with incomplete info than not at all.
Next steps
If you’ve never reported a fake drug before, start now. Save this page. Print the FDA MedWatch form. Keep a pen and camera handy. The next time you see something wrong-don’t ignore it. Don’t assume someone else will report it. You’re the first line of defense. And in the fight against counterfeit drugs, every report counts.








Sandeep Jain
bro i bought some Adderall off Instagram last month and the pills were like, totally chalky? i thought i was just tired but then i saw the spelling error on the bottle-‘Adedall’ lol. i threw it out but now i wish i kept it. thanks for the heads up.
roger dalomba
Wow. A whole article about reporting pills. Next you’ll tell us to recycle our expired Tums.
Sophia Daniels
Let me get this straight-we’re supposed to care about some shady online pharmacy selling fake insulin like it’s a national emergency? Meanwhile, the FDA lets Big Pharma hike prices 300% and calls it ‘innovation.’ You report a fake pill? Cool. But the real crime is the system that makes people buy them in the first place. 🙄
Nikki Brown
I’m so disappointed in people who buy drugs off the internet. 😔 You’re not just risking your life-you’re enabling criminals. And now you want the government to clean up your mess? Grow up. 🙃
Brittany Fuhs
Why are we even talking about WHO and EU reports? If you’re not in America, you shouldn’t be taking American meds anyway. This is OUR system, OUR pills, OUR responsibility. Stop outsourcing your safety.
Erwin Asilom
The FDA MedWatch portal is underutilized. According to 2023 internal metrics, only 12% of reported counterfeit cases include photographic evidence, despite the 89% faster processing rate. Proper documentation-lot numbers, NDC, timestamped photos-is not optional; it’s the cornerstone of traceability. Please, for the love of public health, take the photo.
sakshi nagpal
As someone from India, I’ve seen counterfeit medicines sold in local pharmacies with fake packaging that looks almost identical to the real thing. I reported a fake diabetes pill last year after my uncle had a seizure. The FDA didn’t respond, but the Indian drug authority shut down a small lab in Mumbai within weeks. Reporting matters-even if it feels like shouting into the void. We’re not just protecting ourselves-we’re protecting the global supply chain.
Peter sullen
It is imperative to underscore, with the utmost gravity and procedural rigor, that the act of reporting counterfeit pharmaceuticals constitutes a non-negotiable, systemic imperative-indeed, a bioethical obligation-within the broader framework of pharmacovigilance, public health infrastructure resilience, and transnational regulatory compliance. Failure to adhere to this protocol, whether through negligence, apathy, or misinformation, constitutes a catastrophic dereliction of civic duty, and may, in aggregate, precipitate cascading failures in therapeutic efficacy, patient safety, and the integrity of the global pharmacopeia. Please, for the love of science, and the sanctity of the pill, document. Report. Follow up.