Bioequivalence: What It Means for Generic Drugs and Your Health
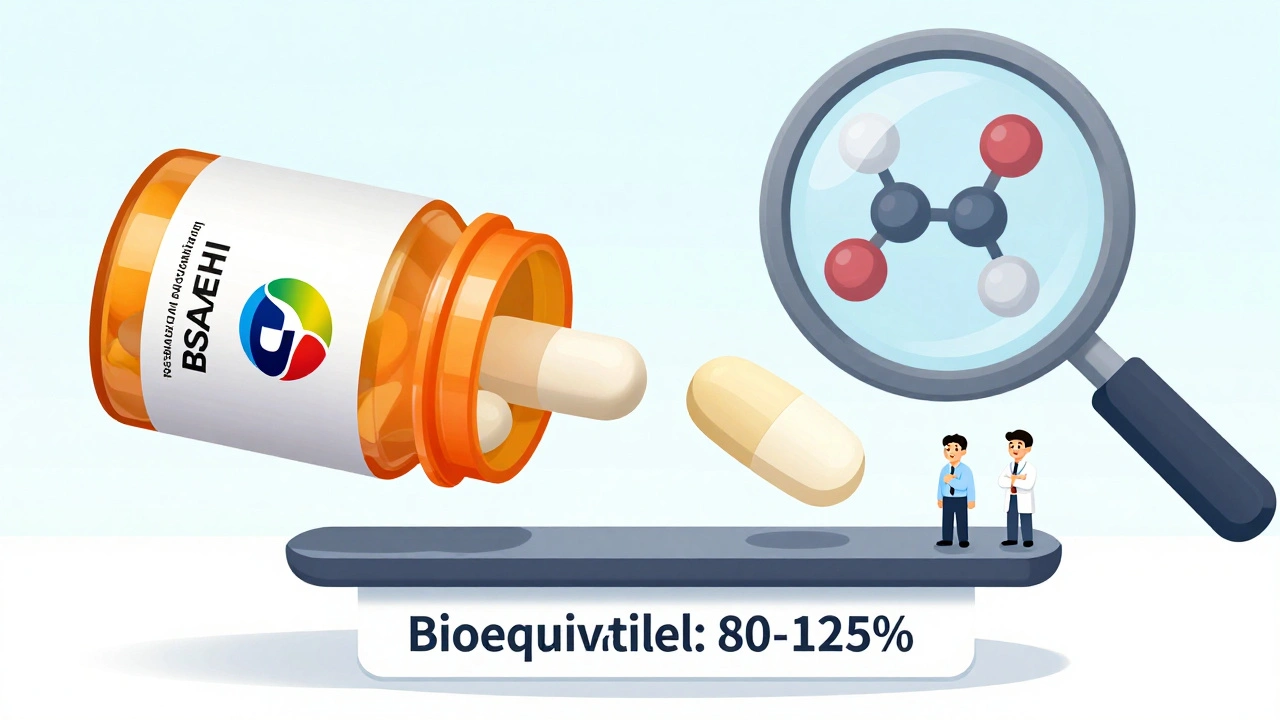
When you pick up a generic pill, you want to know it does the same thing as the brand-name version. That’s where bioequivalence, the scientific standard that proves two drug products release the same amount of active ingredient into your bloodstream at the same rate. Also known as therapeutic equivalence, it’s the reason your pharmacist can swap your brand-name blood pressure pill for a cheaper generic without your doctor needing to rewrite the prescription. Without bioequivalence, you’d be gambling every time you filled a generic prescription.
It’s not just about cost—it’s about control. If your levothyroxine, the standard treatment for hypothyroidism isn’t bioequivalent across brands, your TSH levels could swing unpredictably. That’s why the FDA requires generics to match the brand within a narrow 80–125% range for both peak concentration and total exposure. The same rule applies to antihypertensive combination generics, like pills that combine lisinopril and hydrochlorothiazide. If one generic version absorbs slower than another, your blood pressure might not stay stable. And when you’re managing chronic conditions, stability isn’t optional—it’s life-saving.
Bioequivalence doesn’t mean the pills look the same. They can have different fillers, colors, or shapes. But the active ingredient? It has to behave the same in your body. That’s why a 500mg generic ibuprofen shouldn’t cause more stomach upset than the brand, and why switching from one generic to another for your antibiotics, like azithromycin used in empyema treatment, shouldn’t suddenly make your infection worse. The science behind it is simple: measure how much drug gets into your blood, and how fast. If the curves match, it’s bioequivalent.
But here’s the catch—not all generics are created equal in real life. Some patients report differences even when the numbers say they’re identical. That’s because bioequivalence is tested in healthy volunteers, not people with liver disease, kidney issues, or complex drug regimens. If you’re on multiple meds, like iron with levothyroxine or protein-rich meals with levodopa, your absorption can change. That’s why tracking how you feel matters as much as the lab results.
What you’ll find below is a collection of real-world guides that tie directly to bioequivalence—not just the theory, but the impact. From how to spot when a generic isn’t working for you, to why insurance pushes certain combo pills over others, to how drug shortages force switches that can disrupt your routine. These aren’t abstract science papers. They’re stories from people who’ve been there: the senior whose blood pressure spiked after a generic switch, the diabetic whose insulin cost dropped 80% but didn’t control sugar as well, the patient who learned to read their Rx label to catch hidden changes. This is what bioequivalence looks like when it hits your medicine cabinet.
Clinical outcomes data shows generic drugs are just as effective as brand-name medications for nearly all conditions. Providers can confidently prescribe generics to improve adherence, reduce costs, and maintain patient outcomes.
Generic medications save money but aren't always identical in how they affect your body. For some drugs, even small differences in absorption can cause serious side effects-especially for people on thyroid, seizure, or blood-thinning meds.
The FDA ensures generic drugs work the same as brand-name versions through strict bioequivalence testing, manufacturing standards, and a rigorous approval process. Over 90% of U.S. prescriptions use generics - all proven safe and effective.